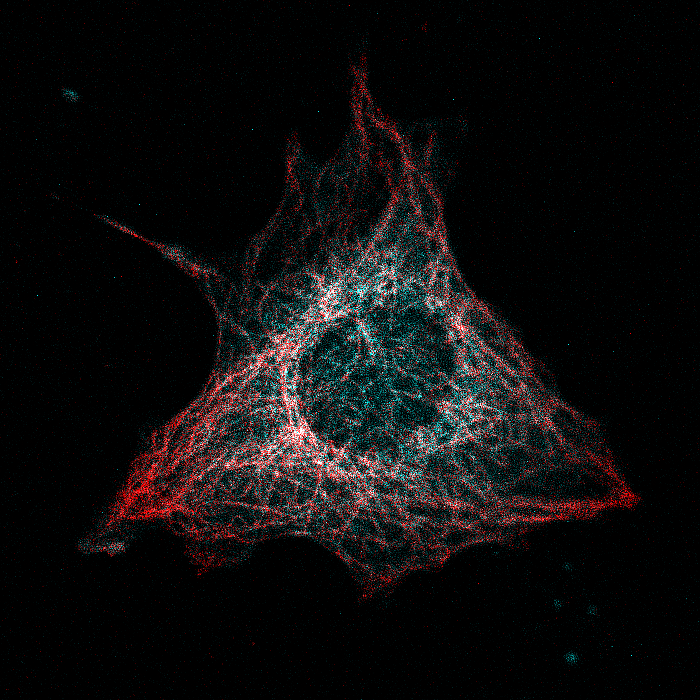
Our lab is interested in uncovering novel signal transduction networks in normal immune cells and in cancer cells. Understanding detailed mechanisms of protein regulation through the formation of multiprotein complexes or through post-translational modification has enabled us to explore the therapeutic potential of signaling molecules in human disease.
Interested and motivated postdoctoral fellows are highly encouraged to contact the lab and graduate students can apply through the Department of Medical Biophysics or the Department of Immunology at the University of Toronto.
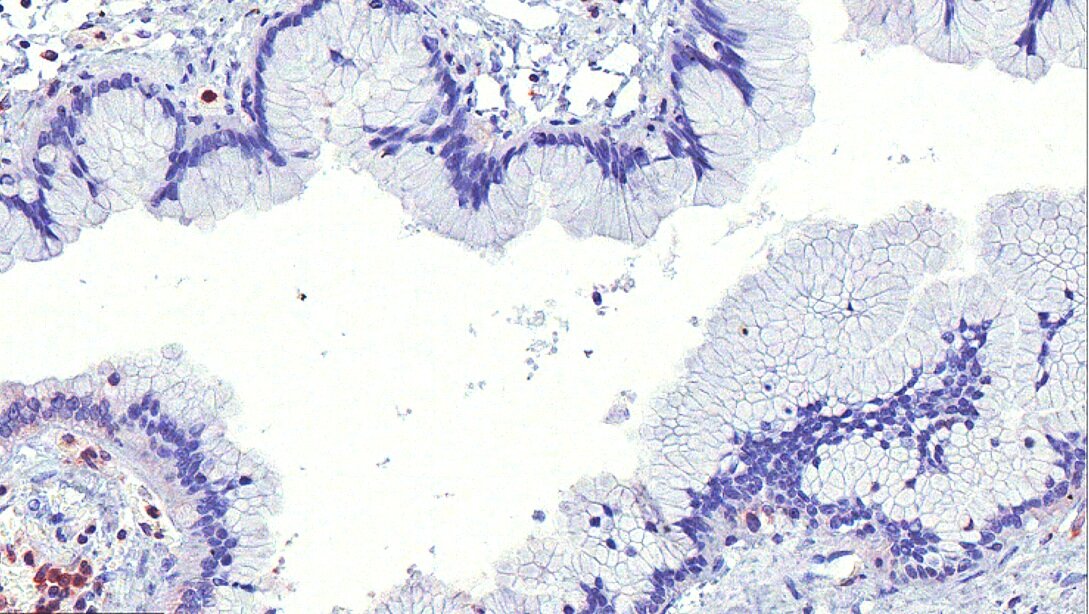
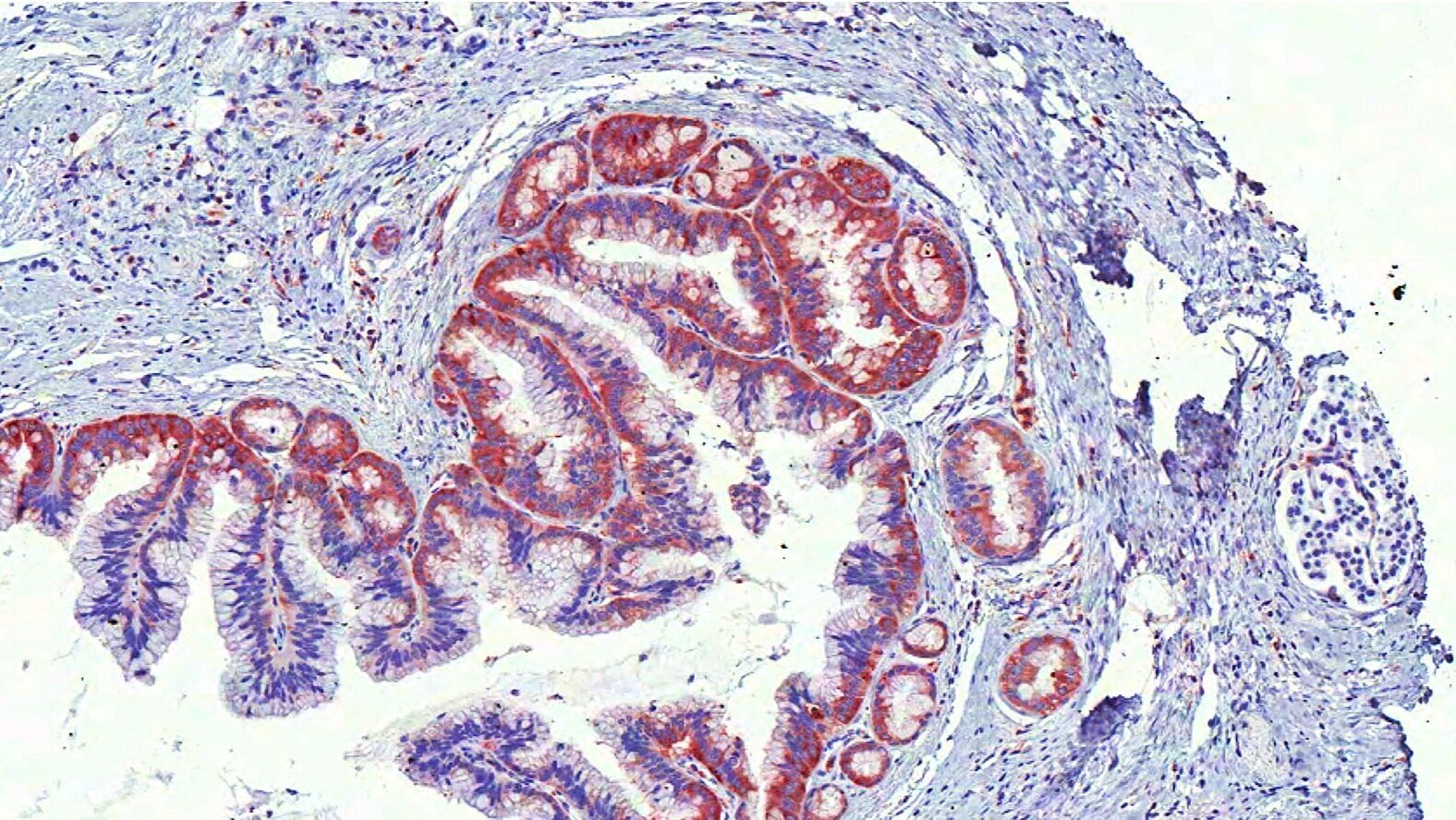
Emergent Vulnerabilities in Ovarian Cancer
As cancer cells evolve, they acquire new fitness properties through genetic mutation that ensures their survival and proliferative potential. However, the acquisition of new fitness properties is often associated with the acquisition of genomic, proteotoxic, metabolic, immunoreactive and oxidative stress, stress states that must be mitigated for the cancer cells to survive. We are systematically identifying the emergent adaptation pathways required for ovarian cancer cell survival using functional shRNA and CRISPR synthetic lethal screens.
We have recently identified an essential autocrine loop involving the secreted protein Relaxin and its receptor RXFP1 in ovarian cancer. We are currently developing neutralizing monoclonal antibodies targeting Relaxin and testing their therapeutic potential.
We have identified the tetraspanin CD151 as an essential gene in HGSOC. CD151 is required for the activation of the SRC and FAK pathways in ovarian cancer cell lines of mesenchymal phenotype; is expressed in a high percentage of primary ovarian cancer tumors; and is biomarker of poor clinical outcome.
Metabolic Targeting of Solid Tumours
We have identified several integrated stress kinases including the amino acid sensor GCN2 as potential therapeutic targets for ovarian cancer and other tumor types. The therapeutic potential of these targets is currently being developed.
We discovered that ovarian cancer cells are highly dependent on the mRNA quality control nonsense mediated decay (NMD) pathway. We have shown that NMD regulates the immuopeptidome on ovarian cancer cells suggesting that inhibition of NMD may be a way to enhance the immunogenicity of tumor cells.
We are using genetic and pharmacologic approaches to uncover the mechanistic basis of these emergent essential signaling pathways.
Regulation of the Rho guanine nucleotide exchange factor GEF-H1
We have discovered that the RHOA guanine nucleotide exchange factor GEF-H1 is sequestered in an inhibited state on microtubules by the dynein motor complex. GEF-H1 release and activation is critically important for the formation of actin contractile structures and the coordinated activation of the MAPK and YAP signaling pathways. These observations demonstrate that cell signaling is critically contingent on the state of cytoskeleton elements a point of control that can be lost in cancer cells.
Modeling Genetic Autoinflammatory Syndromes
We have studied Cherubism, a dominantly inherited disorder leading to facial dysmorphia and activation of immune cells. Mutations in the adapter protein 3BP2 mutated in Cherubism patients leads to hyperactivation of Src, Abl, Syk tyrosine kinases and the guanine nucleotide exchange factor Vav. 3BP2 protein levels are negatively regulated by Tankyrase and the ubiquitin ligase RNF146. Our knockout studies have shown that 3BP2 is required for the activation of T cells, B cells, and neutrophils. We have recently shown loss of 3BP2 control leads to inflammatory bowel syndrome, macrophage activation, inflammatory cytokine production because of Toll like receptor 2 hyperactivation. We are currently studying the role of 3BP2 in macrophage function, phagocytosis and the uptake of oxidized lipid particles by scavenger receptors such as CD36.
We are currently studying the mechanism underlying a recently described autoinflammatory syndrome characterized by neonatal onset of pancytopenia, autoinflammation, rash, and hemophagocytic lymphohistiocytosis called NOCARH. This disorder has mutations in the C-terminus of CDC42.
Latest publications
Translational Control by 4E-BP1/2 Suppressor Proteins Regulates Mitochondrial Biosynthesis and Function during CD8+ T Cell Proliferation
Tankyrase represses autoinflammation through the attenuation of TLR2 signaling
Tankyrase regulates epithelial lumen formation via suppression of Rab11 GEFs
Inhibition of relaxin autocrine signaling confers therapeutic vulnerability in ovarian cancer